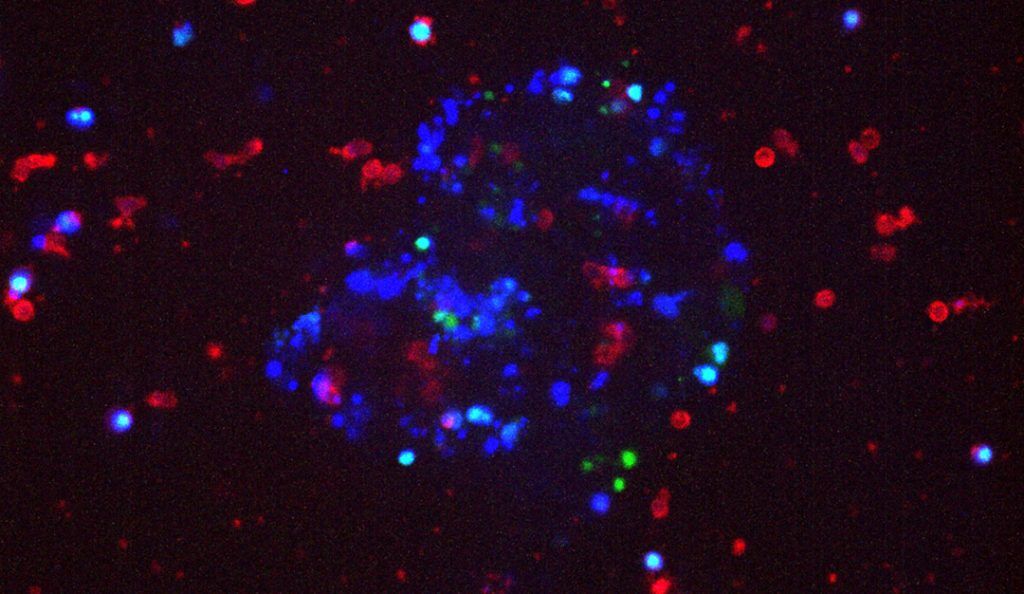
Study is the first to show that breast cancer cells can manipulate natural killer immune cells and opens up a promising new avenue for research
In a first-of-its-kind study published in the Journal of Cell Biology, BCRF researchers Drs. Isaac Chan, Andrew Ewald, Elizabeth Jaffee, and colleagues reported that they discovered a means by which breast cancer cells metastasize by overtaking immune cells—and that the process can be targeted and blocked.
Metastasis—when cancer spreads to other regions of the body—is the leading cause of breast cancer deaths and has no cure. At this advanced stage, breast cancers have become resistant to many of the drugs used to treat them and have learned how to evade the immune cells that are supposed to kill them.
Natural killer (NK) cells are a type of white blood cell that is critical to the innate immune system, as they’re able to react to a harmful pathogen without having previous exposure to it. NK cells play a role in tumor immunosurveillance and have potent anti-tumor and anti-metastasis activity. Tumor cell detection causes NK cells to be activated, attack the tumor cells, and induce cell death. To form metastases, breast cancer cells have to evade attacks from NK cells. What isn’t well understood, however, is how they do this. In research supported by BCRF, Dr. Chan and his colleagues, showed that breast cancer cells trick NK cells into not attacking them and instead co-opt them to promote metastasis.
Determining how NK cells fight breast cancer metastasis
Using a novel 3-D cell culture method, developed in the Ewald laboratory, the researchers observed the interactions between NK cells and invasive breast cancer cells in real time. In these experiments, they studied breast cancer cells that express the protein cytokeratin-14 (K14+), which are a specialized type of cell that lead invasion and metastasis in most breast cancer subtypes. The researchers determined the molecular mechanism by which NK cells limit early metastasis: NK cells activate an enzyme in K14+ cells that causes them to die.
How breast cancer still thwarts NK cells
Why, then, do NK cells not completely stop metastases from forming? Drs. Chan, Ewald, and colleagues speculated that the K14+ breast cancer cells become resistant to NK cell attack. They observed that if the invading K14+ cells and NK cells were left together for three to four days, the K14+ cells were able to partially overcome the NK cells’ attack.
To further explore this, the researchers isolated NK cells that had already been exposed to tumors and found that they had lost their cancer-fighting activity. Strikingly, the team found that breast cancer cells can rapidly reprogram healthy NK cells. Instead of preventing breast cancer metastasis, they promoted it.
Using molecular profiling and computational analyses the researchers were able to pinpoint the signals responsible for co-opting the NK cells.
Reversing NK cell reprogramming
Blocking the signals that allow K14+ cells to reprogram NK cells restored the NK cells’ ability to fight tumors. The researchers identified two potential targets called TIGIT and KLRG1 and showed that they can be blocked with targeted antibodies to curb invasion of K14+ cells. Additionally, they were able to demonstrate that in combination with DNMT inhibitors, used to treat other types of cancer, that metastasis is even further reduced. Reversing reprogramming of NK cells could be a promising step to slow or stop metastasis in the clinic.
What this means for patients
Understanding how immune system NK cells and breast cancer cells interact—and how reprogrammed NK cells alter those interactions to trick the immune system—opens up a new set of novel drug targets to potentially prevent or treat metastasis. While years away from benefitting patients, this approach, in combination with other types of therapies, could be a promising clinical strategy to treat and fight cancer.
“BCRF funding enabled us to pursue this exciting new direction years before we could have secured funding from the federal government,” Dr. Ewald said. “We are very excited that our discovery identifies a therapeutic concept that could drive new immunotherapy trials for breast cancer patients.”